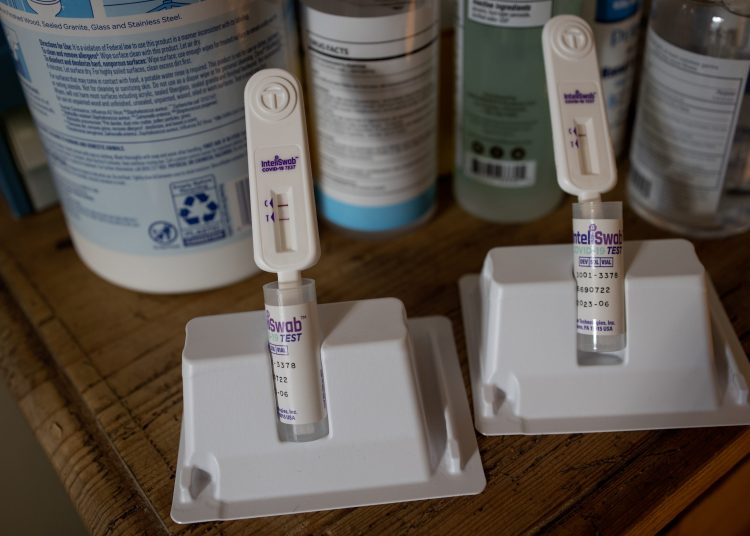
Rapid antigen tests may be slightly susceptible to detecting the highly contagious omicron variant and could steer to results that are “false negative,” the Food and Drug Administration announced Tuesday. A preliminary study by the FDA, in affiliation with the National Institutes of Health’s Rapid Acceleration of Diagnostics program, utilized samples from patients confirmed to be infected with the omicron strain of the virus to examine the performance of at-home tests, also known as “antigen” tests. The agency explained early data indicates that antigen tests “do detect the omicron variant but may have reduced sensitivity,” implying it’s feasible that these tests could miss an infection. Prior testing by RADx directed on heat-inactivated virus samples, which are patient specimens ascertained to be infected with the omicron variant that has been heat-treated so that the virus is no longer living. Presently-available antigen tests were able to detect the mutated strain “with similar performance” to other variants in that research.
Merck’s new COVID drug molnupiravir gets FDA consideration : Shots – Health News
However, the agency reports that live virus is the best way to assess true test performance, while “heat-inactivated samples are the best available option when patient samples with the live virus are not available.”The FDA stopped short of instructing people to avert using antigen tests and did not prescribe the brands of tests used in the study. The agency also gave emphasis to the findings being based on laboratory data and not the more credible clinical study evaluations, which are presently ongoing. “The FDA continues to authorize the use of these tests as directed in the authorized labeling and people should continue to use them by the instructions included with the tests,” the agency explained in a statement. “Antigen tests are generally less sensitive and less likely to pick up very early infections compared to molecular tests.”